Long-Acting Injectables Transform HIV Prevention and Treatment
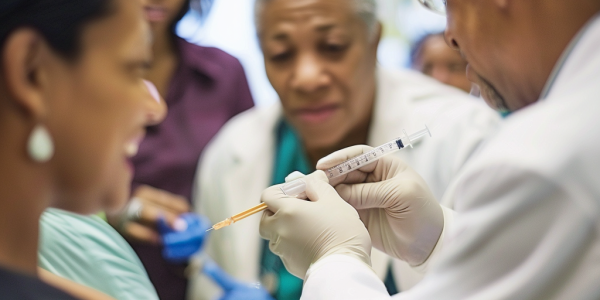
Long-acting injectable medications are transforming HIV prevention and treatment, offering a significant alternative to daily pills. Approved by the EMA and FDA, these injectables like cabotegravir and rilpivirine provide convenient dosing options for individuals at risk or living with HIV, enhancing adherence and reducing stigma. Discover how these innovative therapies are paving the way for improved health outcomes in the fight against HIV.
CDC Investigates Botulism-like Illnesses Linked to Botox Injections
The CDC is investigating botulism-like illnesses in multiple states linked to non-medical Botox injections. Patients in Tennessee and Illinois experienced symptoms after receiving injections from unlicensed providers. Authorities warn of the dangers of counterfeit products and urge the public to seek Botox treatments only from licensed medical professionals.
Woman Nearly Dies After Botox Injections for Migraine Treatment
Alicia Hallock’s harrowing experience with Botox injections for migraines led to her ICU admission. The injections left her partially paralyzed and suffering from dysphagia, requiring a feeding tube for nutrients. Hallock’s recovery journey was shared on social media, emphasizing the severity of her experience with Botox complications.
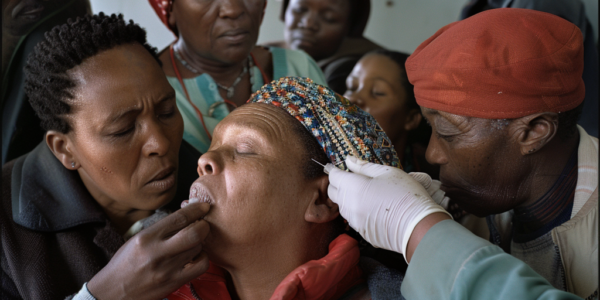
New HIV Prevention Injection Offered in South Africa
A groundbreaking new HIV prevention injection is now being offered to a limited number of individuals in South Africa, providing two months of protection with a single shot. Administered outside of clinical trials, the injection contains a long-acting formulation of the antiretroviral drug cabotegravir (CAB-LA), offering a more convenient and less painful alternative to traditional methods of prevention. Select individuals in South Africa have access to it through pilot projects such as the FAST PrEP study, aiming to introduce the injectable and gather valuable insights for future large-scale implementation.