FDA Recalls 28 Beverages in 2024 Due to Undisclosed Drugs, Bacteria, and Chemicals
The FDA has recalled 28 beverages in 2024 due to undisclosed drugs, bacteria, and harmful chemicals. From arsenic in Martinelli’s Apple Juice to bacteria in Fiji Water, consumers are urged to be cautious. Stay informed on the latest recalls including Schweppes Zero Sugar Gingerale and PepsiCo’s Mug Root Beer.
FDA Lifts Marketing Ban on Juul Vaping Products
The FDA has lifted the marketing ban on Juul’s vaping products, but the fate of these products is still pending a final decision. Juul’s products will continue to be available for purchase while undergoing a scientific review process. This move signifies a pivotal moment in the evaluation process, emphasizing the need for FDA authorization for all e-cigarette products, including Juul’s. Stakeholders await further developments to determine the future status of Juul’s products in the market.
Expert Panel Votes Against MDMA Therapy for PTSD Treatment
An expert panel in the United States has voted against the use of MDMA therapy for treating PTSD, citing concerns about the drug’s efficacy. Despite promising results from trials, panelists raised doubts about the long-term benefits and potential risks associated with the treatment. The FDA is expected to make a final decision by August 11, while Australia has approved the use of MDMA for therapy sessions.
Cionic’s Neural Sleeve Shows Positive Impact on Multiple Sclerosis Patients in Real-World Study
Discover the positive impact of Cionic’s Neural Sleeve technology on patients with multiple sclerosis. A recent study highlighted improvements in walking challenges, showcasing enhanced foot and ankle mobility. CEO Jeremiah Robison emphasizes the importance of tailored technology for neurologic conditions, offering hope for improved quality of life.
FDA recalls nearly 1.9 million bottles of Fiji water in New York due to contamination concerns
The FDA has issued a recall for nearly 1.9 million bottles of Fiji Natural Artesian Water in New York due to concerns over manganese and bacterial contamination. Consumers are urged to stop using the affected water bottles and seek guidance from the appropriate authorities.
FDA issues Class I recall for t:connect iPhone app due to battery drain glitch in insulin pump
The FDA has issued a Class I recall for the t:connect iPhone app due to a software glitch that drained the batteries of the t:slim X2 insulin pump, leading to potential pump shutdowns. Consumers are advised to monitor pump battery levels closely and update to version 2.7.1 or later to prevent unexpected shutdowns and health risks associated with insulin delivery disruptions.
Groundbreaking Gene Therapy for Sickle Cell Disease Shows Promising Results
Researchers at Children’s Hospital of Philadelphia (CHOP) have published final results of a clinical trial for gene therapy targeting sickle cell disease. The study showed 96.7% of patients did not experience VOCs for at least a year, leading to FDA approval of CASGEVY in December 2023. This groundbreaking therapy, developed using CRISPR technology, has shown promising outcomes in preventing pain episodes and improving quality of life for patients.
FDA Clears MamaLift Plus for Postpartum Depression Treatment
Curio Digital Therapeutics receives FDA clearance for MamaLift Plus, a prescription digital therapeutic device for postpartum depression. The device, supported by the SuMMER trial, offers evidence-based therapies through a smartphone or tablet for convenient treatment.
Groundbreaking Drug Development May Extend Lifespan of Dogs
Loyal, a biotech company, is making strides in developing a drug to extend the lifespan of large-breed dogs. The FDA’s recent approval marks a significant step towards potentially increasing the longevity of our furry friends. By targeting IGF-1 signaling, the drug aims to slow down the aging process in dogs, offering hope for a future where our beloved canine companions can live longer, healthier lives.
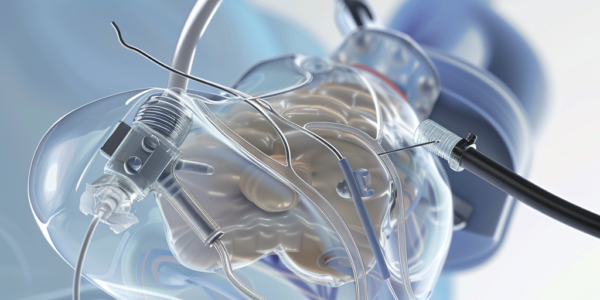
FDA Issues High-Level Alert for Impella Left-Sided Heart Pumps
The FDA has issued a high-level alert regarding the Impella left-sided heart pumps, which have been associated with 49 deaths and 129 injuries. Despite the recall, the device will remain available on the market. Abiomed, the manufacturer of the device, has released new instructions for the pump in response to the FDA’s alert. The FDA’s notice pertains to 66,390 devices distributed in the US over a two-year period starting from October 10, 2021. This development underscores the importance of adhering to proper usage guidelines for medical devices, particularly those designed to support critical bodily functions.