Cystic fibrosis, a hereditary disease that has been incurable, is now seeing new hope with the approval of triple combination therapy for children in addition to adults. A recent observational study conducted by Charité – Universitätsmedizin Berlin reveals significant improvements in the health status of school-aged children with cystic fibrosis following this new treatment.
People with cystic fibrosis typically experience the buildup of thick, viscous mucus in their lungs, leading to various respiratory issues. Traditional treatments focus on managing symptoms through daily inhalations, antibiotics, and physical therapy. However, the introduction of triple combination therapy, consisting of elexacaftor, tezacaftor, and ivacaftor, aims to address the root cause of the disease.
Prof. Mirjam Stahl, the head of the Division of Cystic Fibrosis at Charité, expresses optimism about the therapy’s availability for children, emphasizing its positive impact on young patients. The therapy, administered as a pill, was initially approved for children aged six and older in 2022, with further approval for children as young as two years in 2023.
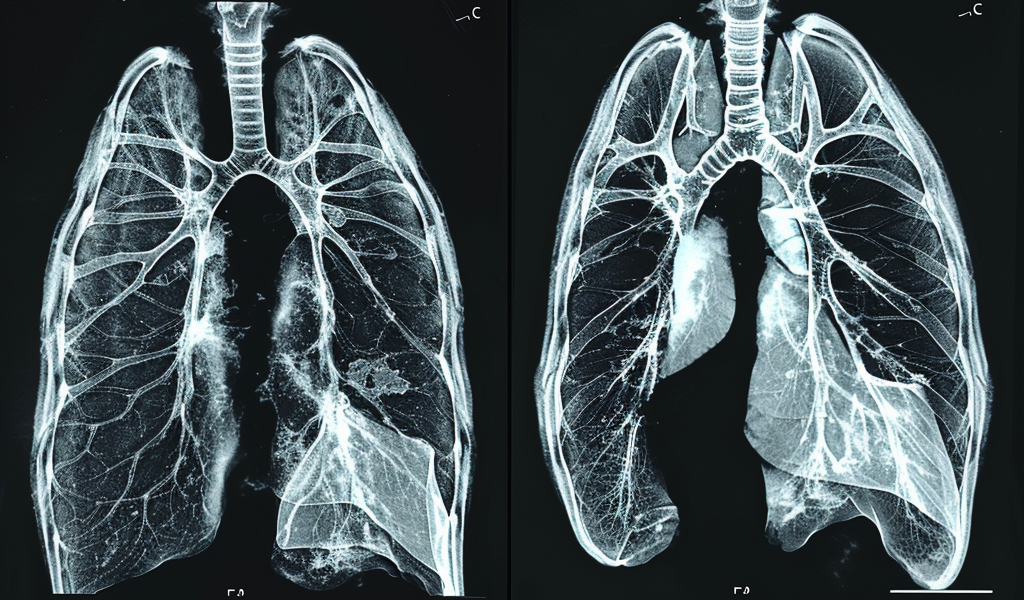
The study showcases MRI images of a child with cystic fibrosis before and after receiving triple combination therapy, highlighting the reversal of mucus deposits, bronchial widening, and thickening of bronchial walls in the lungs. These promising results offer hope for improved outcomes and enhanced quality of life for individuals with cystic fibrosis.