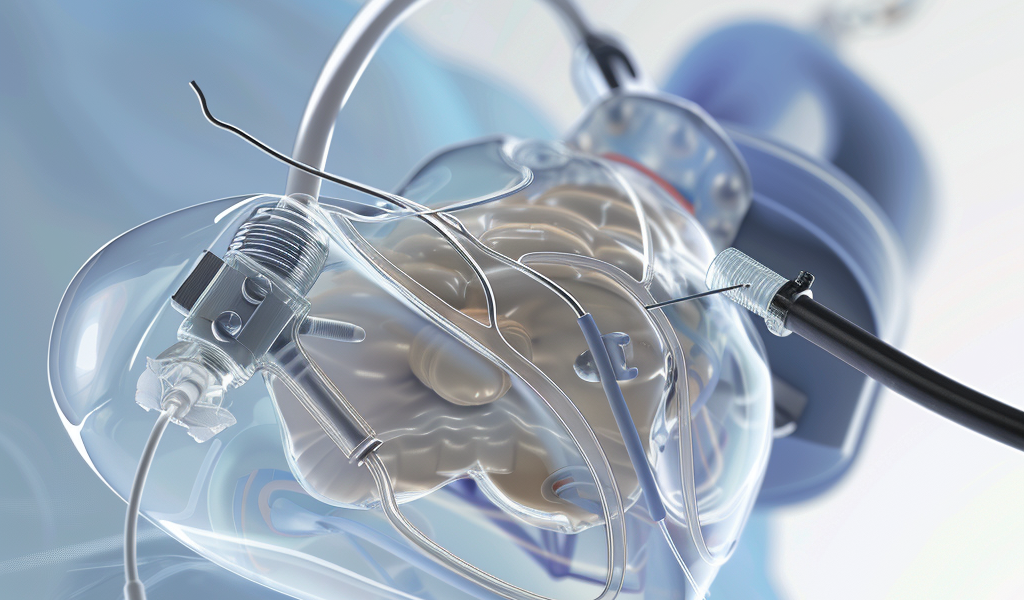
The US Food and Drug Administration (FDA) has issued a high-level alert regarding the Impella left-sided heart pumps, which have been associated with 49 deaths and 129 injuries. These pumps are utilized to provide temporary support to a patient’s heart during high-risk procedures or following a severe heart attack. However, the FDA has cautioned that improper use of the pumps could result in puncturing the heart’s left ventricle wall.
Abiomed, the manufacturer of the device, has released new instructions for the pump in response to the FDA’s alert. The FDA classified this action as the most serious type of recall due to the potential for serious injuries or fatalities if the device is misused. The agency highlighted that the use of affected pumps may lead to severe health consequences, including hypertension, lack of blood flow, and death.
Despite the recall, the device will remain available on the market. The FDA’s notice pertains to 66,390 devices distributed in the US over a two-year period starting from October 10, 2021. Although the device received FDA approval in 2008, the recent alert has raised concerns about its safety.
The pumps consist of a catheter with a small hook at the end, which is threaded through the blood vessels and into the heart’s left ventricle. Johnson & Johnson, which acquired Abiomed in 2022, emphasized that the notification does not entail a device removal and assured that the Impella heart pumps will continue to be available for patients.
According to the FDA, Abiomed initially disclosed the risk of heart perforation during pump insertion in a technical bulletin in October 2021 but failed to inform the FDA at the time. Subsequently, the agency conducted an inspection of Abiomed’s office in Massachusetts in early 2023 and issued a warning letter to the company in September, citing a failure to update the FDA on the risk of heart perforation. This prompted Abiomed to issue an ‘Urgent Medical Device Correction letter’ late last year, containing revised instructions for the correct use of the heart pump.
This development underscores the importance of adhering to proper usage guidelines for medical devices, particularly those designed to support critical bodily functions. As the FDA continues to monitor the situation, healthcare providers and patients should remain vigilant about the potential risks associated with the Impella left-sided heart pumps.