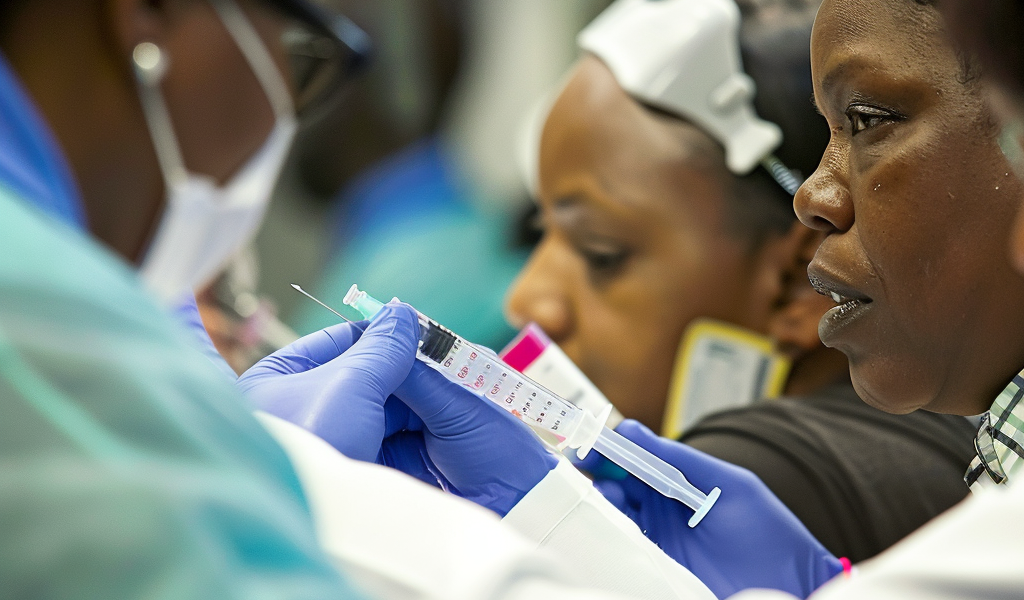
In a recent development in HIV treatment, England has started providing injectable HIV antiretroviral therapy (ART) to patients, with hundreds already receiving the two-monthly injections. The British HIV Association’s Spring Conference in Birmingham unveiled some initial data from the rollout, indicating overall effectiveness and positive patient feedback, although there have been a few cases of withdrawal.
Despite the promising start, concerns have arisen due to two reported instances of virological failure with drug resistance. This mirrors findings from scientific trials where 1% of participants experienced virological failure, leading to resistance to one or both drugs used in the therapy. This resistance could potentially limit future treatment options.
The injectable ART utilizes long-acting forms of cabotegravir (Vocabria) and rilpivirine (Rekambys), administered as two separate injections. The initial doses are given a month apart, followed by injections every two months. Patients also have the option of a month’s worth of tablets before starting the injections to ensure tolerance.
While the injections have shown efficacy and patient preference, the emergence of drug resistance in some cases is a cause for concern. Researchers are working to understand how virological failure can occur despite apparent adherence to the treatment regimen. This ongoing investigation aims to improve the effectiveness and reliability of injectable HIV therapy.
Stay informed about the latest developments in HIV treatment and learn more about the challenges and advancements in injectable medication to combat the virus.