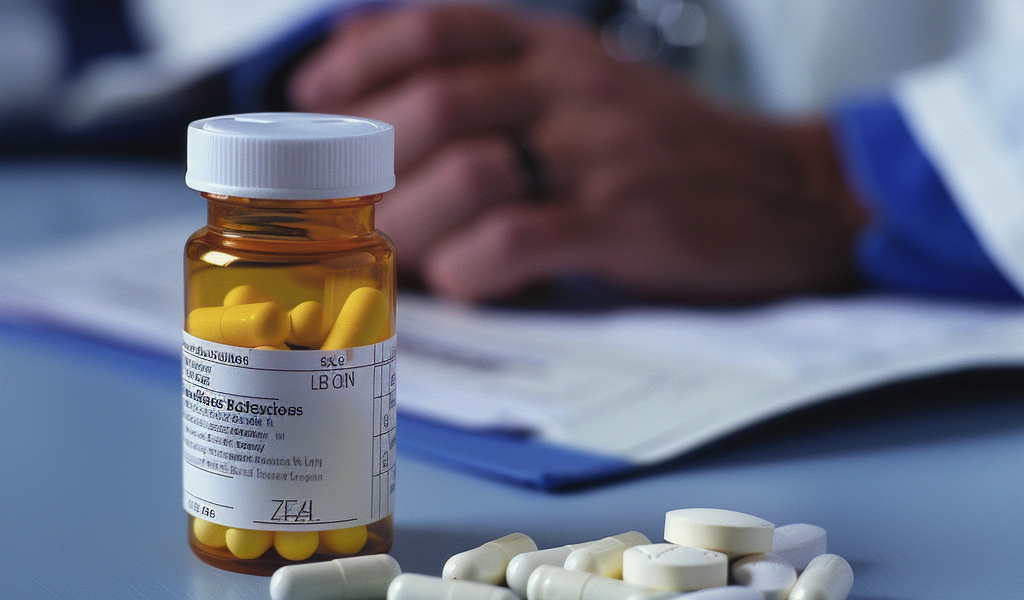
The Food and Drug Administration (FDA) has intensified its alert regarding a recall affecting over 7,100 bottles of Duloxetine Delayed-Release Capsules, a commonly prescribed antidepressant. This escalation to a Class II recall signifies a significant concern for public health, as it indicates that exposure to the affected product may lead to temporary or medically reversible health issues, though the risk of severe consequences remains low.
Duloxetine, widely recognized under the brand name Cymbalta, is primarily utilized in the treatment of depression, anxiety, and various mood disorders. It belongs to a category of medications known as selective serotonin/norepinephrine reuptake inhibitors (SNRIs), which work by balancing chemicals in the brain that affect mood and emotional state.
The FDA’s decision to raise the recall classification stems from the discovery of an increased level of a potentially harmful chemical, N-nitroso-duloxetine, in the recalled capsules. This chemical is classified as a probable human carcinogen, raising concerns about its implications for long-term health.
Patients currently taking Duloxetine are advised not to discontinue their medication abruptly. The FDA emphasizes the importance of consulting healthcare professionals before making any changes to prescribed treatments. This guidance is crucial, as sudden cessation of antidepressants can lead to withdrawal symptoms and a potential relapse of the underlying condition.
In response to the recall, the FDA is actively investigating the origins of the contamination to ensure that similar issues do not arise in the future. The agency is committed to safeguarding public health and ensuring the safety of medications available to consumers.
This recall is part of a broader trend of heightened scrutiny over pharmaceutical products, particularly those that have been linked to health risks. The FDA’s actions reflect an ongoing commitment to monitoring drug safety and efficacy, ensuring that patients are informed of potential risks associated with their medications.
As the investigation into the source of the contamination continues, patients are encouraged to stay informed about the medications they are prescribed and to maintain open communication with their healthcare providers. It is essential for individuals to understand the implications of any recalls and to take proactive steps in managing their health.
In addition to the Duloxetine recall, the FDA has been involved in various other safety alerts and recalls in recent months, highlighting the importance of vigilance in the pharmaceutical industry. This includes recalls of food products and other medications that pose health risks to consumers.
The FDA’s proactive approach in addressing these issues aims to enhance the safety and well-being of the public. By raising awareness and providing clear guidelines, the agency seeks to mitigate risks associated with pharmaceutical products and ensure that patients receive safe and effective treatments.
For individuals affected by this recall or those with questions about their medications, it is advisable to consult with healthcare professionals for personalized advice and support. Staying informed and taking appropriate actions can help ensure health and safety in light of this recent development.