A new model from PPPL researchers explains the production of black silicon using fluorine gas, enhancing its application in solar cells and marking a new direction in quantum chemistry research.
Researchers at the U.S. Department of Energy’s Princeton Plasma Physics Laboratory (PPPL) have developed a new theoretical model explaining one way to make black silicon, an important material used in solar cells, light sensors, antibacterial surfaces, and many other applications.
Black silicon is made when the surface of regular silicon is etched to produce tiny nanoscale pits on the surface. These pits change the color of the silicon from gray to black and, critically, trap more light, an essential feature of efficient solar cells.
While there are many ways to make black silicon, including some that use the charged, fourth state of matter known as plasma, the new model focuses on a process that uses only fluorine gas. PPPL Postdoctoral Research Associate Yuri Barsukov said the choice to focus on fluorine was intentional: The team at PPPL wanted to fill a gap in publicly available research. While some papers have been published about the role of charged particles called ions in the production of black silicon, not much has been published about the role of neutral substances, such as fluorine gas.
“We now know — with great specificity — the mechanisms that cause these pits to form when fluorine gas is used,” said Barsukov, one of the authors of a new paper about the work. “This kind of information, published publicly and openly available, benefits us all, whether we pursue further knowledge into the basic knowledge that underlines such processes or we seek to improve manufacturing processes.”
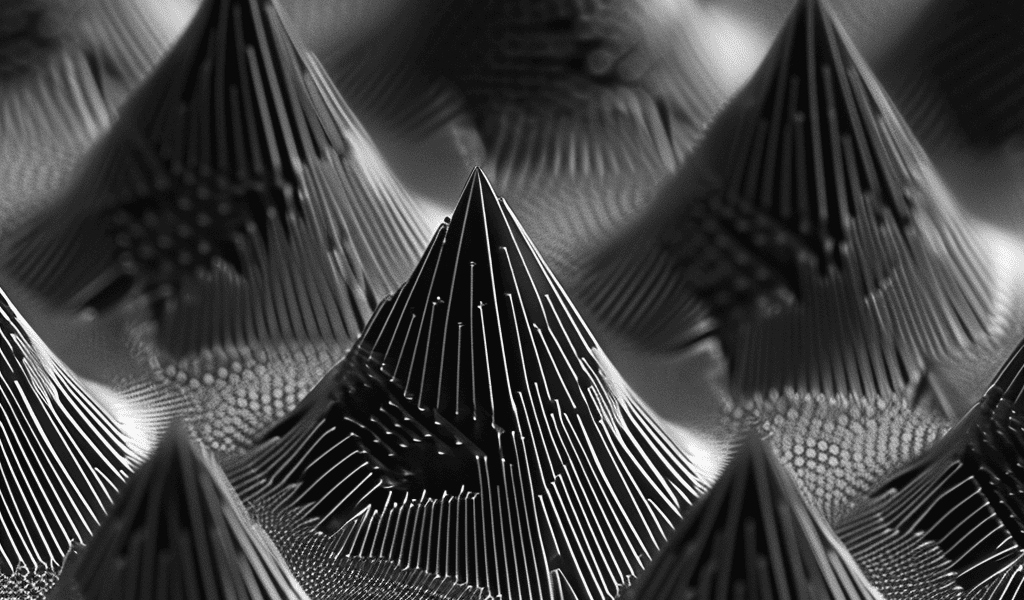
Fluorine gas etches the surface of silicon into a series of angular peaks that, when viewed with a powerful microscope, look much like the pyramid pattern in the artist’s concept shown above. Researchers at PPPL have now modeled how these peaks form in silicon, creating a material that is highly light absorbent. Credit: SciTechDaily.com