Novel UV Broadband Spectrometer Improves Air Pollutant Analysis
Sunlight plays a significant role in chemical processes, with its high-energy UV radiation being strongly absorbed by all materials and triggering photochemical reactions in the air. One well-known example is the formation of ground-level ozone when UV light interacts with nitrogen oxides.
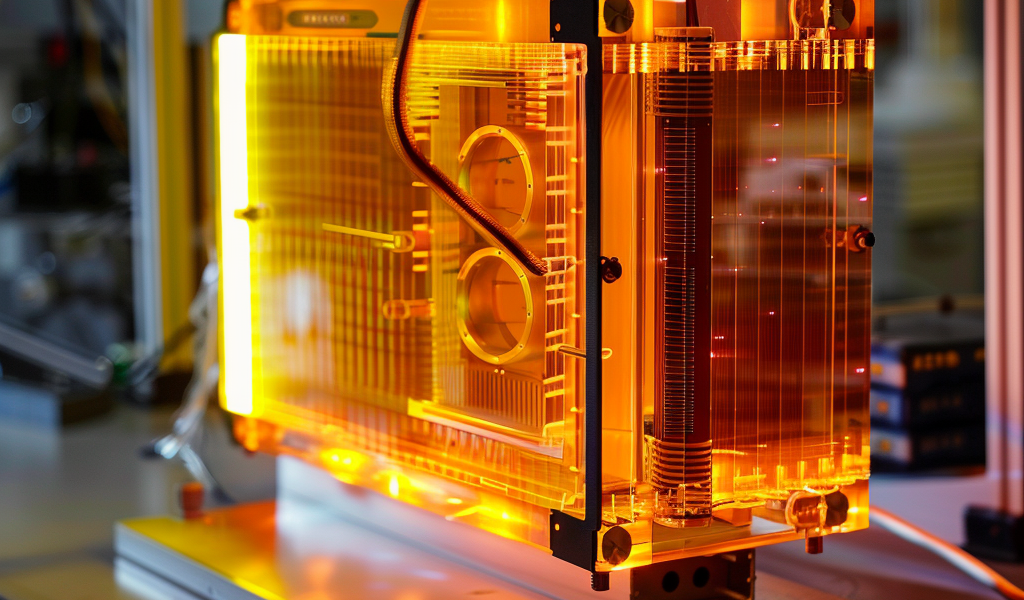
At Graz University of Technology (TU Graz), a research team led by Birgitta Schultze-Bernhardt from the Institute of Experimental Physics has developed the world’s first broadband UV dual-comb spectrometer. This innovative device allows for the continuous measurement of air pollutants and real-time observation of their reactions with the environment.
The team’s work has been published in the journal Optica. Dual-comb spectrometers, which have been in existence for nearly two decades, involve a source emitting light across a broad wavelength range, resembling the teeth of a comb when arranged according to its optical frequencies. When this light passes through a gaseous material sample, the molecules within it absorb some of the light. The altered light wavelengths provide insights into the ingredients and optical properties of the analyzed gas.
What sets the spectrometer developed by Birgitta Schultze-Bernhardt apart is the use of a laser system that emits double light pulses in the ultraviolet spectrum. When this UV light interacts with gas molecules, it electronically excites the molecules and causes them to rotate and vibrate, known as rovibronic transitions, which are unique to each gaseous substance.
The broadband UV dual-comb spectrometer offers three key properties that conventional spectrometers have previously only been able to offer in part:
- A large bandwidth of the emitted UV light, enabling the collection of a wealth of information about the optical properties of gas samples with a single measurement.
- High spectral resolution, which will facilitate the investigation of complex gas mixtures such as Earth’s atmosphere in the future.
- Short measurement times when analyzing gas samples, making the spectrometer suitable for sensitive measurements to observe changes in gas concentrations and the course of chemical reactions.